Abstract
Introduction. NETosis and the formation of neutrophil extracellular traps (NETs) are known to be associated with the pathogenesis of various infectious diseases and thrombosis. However, the extent and clinical significance of NETosis and its released plasma markers in patients with a SARS-CoV2 infection resulting in severe and critical coronavirus disease 2019 (COVID-19) are not fully understood.
Patients and Methods. COVID-19 patients on admission were prospectively enrolled for the study. Patients were classified according to WHO guidelines into asymptomatic (control), mild-moderate (not requiring oxygenation), severe (requiring oxygenation), and critical (multiple organ failure and/or requiring ventilator). Whole blood anticoagulated with EDTA from each patient was collected. Cells were fixed and stained for flow cytometry analysis following incubation with anti-CitrH3 and anti-MPO, and plasmas were used for assessing the soluble NETs.
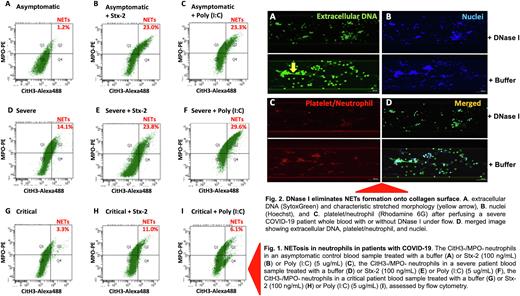
Results. Seventy-six hospitalized patients with PCR positive SARS-CoV2 were categorized into asymptomatic control (n=11), mild-moderate (n=28), severe (n=22), and critical (n=15) groups. Flow cytometry detected only few CitH3+/MPO+ positive neutrophils (undergoing NETosis) in blood of asymptomatic controls on admission. However, the number of CitH3+/MPO+ neutrophil dramatically increased following a 15-minute stimulation with a bacterial Shigatoxin-2 at 100 ng/mL or polyinosinic-polycytidylic acid [Poly (I:C)], a TLR3 agonist at 5 ug/mL for 3 hours (Fig. 1). The number of the CitH3+/MPO+ positive neutrophils in the samples from severe COVID-19 patients was significantly higher than that in asymptomatic controls (p=0.021). Interestingly, the number of the CitH3+/MPO+ positive neutrophils in critical patients was in fact lower than that in severe patients (p=0.013). Moreover, upon stimulation with Poly (I:C), the number of the CitH3+/MPO+ positive neutrophils in asymptomatic patients were more dramatically increased than those in severe/critical patients, suggesting a decrease in neutrophil reserve that could undergo NETosis and fight for infection. Microfluidic shear-based assay and confocal imaging analysis further demonstrated an increased formation of NETs strings under flow in whole blood samples from severe COVID-19 patients compared with those from asymptomatic controls (p=0.012). The NETs strings were sensitive to digestion by DNase I (Fig. 2), suggesting the therapeutic efficacy of DNase I in severe COVID-19 patients. Finally, plasma levels of cell-free DNA and histone-DNA complexes (the soluble markers of NETs in blood) were also more significantly increased in patients with severe and critical COVID-19 than those in the asymptomatic controls.
Disclosures
Zheng:Alexion: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Takeda: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal